In Alice in Wonderland (Chapter III, “A Caucus-Race and a Long Tale”), Alice viewed a race among all animals initiated by the Dodo bird. “… [A]s they began running when they liked, and left off when they liked, so that it was not easy to know when the race was over. However, when they had been running half an hour or so … the Dodo suddenly called out ‘The race is over!’ and they all crowded round it, panting, and asking, ‘But who has won?’ This question the Dodo could not answer without a great deal of thought … At last, the Dodo said, ‘Everybody has won, and all must have prizes.'”
This “Dodo bird verdict” (DBV) — everybody has won — has been applied to a long-time and never-ending debate about which psychotherapy approach is more effective in treating patients in need of psychotherapy (1), psychodynamic psychotherapy (PDPT), cognitive-behavioral psychotherapy (CBT), or one of the many other therapy modalities that exist in between and beyond and claim to be the best for all and everybody. Psychotherapists of all colors and proveniences have a clear answer to this question: Mine is the best because I believe it in and I am good at it. This is a prerequisite to being able to truly help the patient — you have to be convinced to be convincing.
The empirical side of this competition tells a different story. Whenever different psychotherapies were tested in clinical trials against another therapy of the same or different kind or against another control condition (see below), the effects of one therapy was not that much different from another therapy. Meta-analyses of today (2) confirm what Rosenzweig described in the classical DBV paper 80 years ago (1): All psychotherapies operate with similar assumptions, implement similar means, and generate similar results, based on what has been called “common factors” that are immanent to all psychotherapy traditions.
This opens the avenue to two important questions: For one, how much (in terms of claimed percentage), then, is — for each and every psychotherapy modality — specific for this therapy, and how much is common to all? There are empirical answers to this that we will address. The second question is more challenging: If a substantial fraction is “common factors,” how much of this is identical to what has been called the “placebo effect” in other medical therapies, such as drugs, surgery, or manual therapy?
To give an example, in the list of factors discussed to be part of the commonalities (3), “therapy warmth, empathy, acceptance, genuineness, trust” have been identified to drive the placebo response in experimental and clinical trials, e.g. using sham acupuncture, and “changing expectations” and “modeling” have been shown to be mechanisms underlying the placebo effect (4).
On the other hand: In drug trials including a placebo arm, many of these common factors occur incidentally, not as planned interventions, but because one doctor may act this way naturally while another one may not — we have already seen that the sex of the therapist may make a difference. In comparison: in psychotherapy, these common factors are part of the routine, and therapists are trained to act empathic — and empathy can be learned.
From this, we learn: (A) Unspecific factors (regression to the mean, spontaneous symptom variation, Hawthorn effects, and the like) may still be unspecific factors that apply to psychotherapy, as they do in somatic therapy. Furthermore (B), there are genuine placebo effects, neurobiological effects driven by expectations (all patients expect to become better with treatment) and learning (all patients — with a few exceptions, maybe — have had diseases in the past and have been helped. This is medical history we carry with us all the time). And if it is not our own medical history, then it is the medical experience of our proxies that drive our expectation (called “placebo by proxy” (5) – another topic we will have to deal with at some point).
These effects operate irrespective of the type of therapy. At the same time (C) some unspecific factors that are driving the placebo effect in somatic medicine (in drug therapy, surgery, manual therapy, and the like) may become common factors in all psychotherapies, e.g. the intensity of the patient-therapist relationship. And last but not least, (D) the specifics of each and every psychotherapy, as mentioned above; they have been estimated to account for only 10% of psychotherapy success, if meta-analyses are used for comparison (2).
This complex relationship between therapy specific effects, unspecific effects, and placebo effects is illustrated in Figure 1 (modified from (6)), and compared to the situation with drugs.
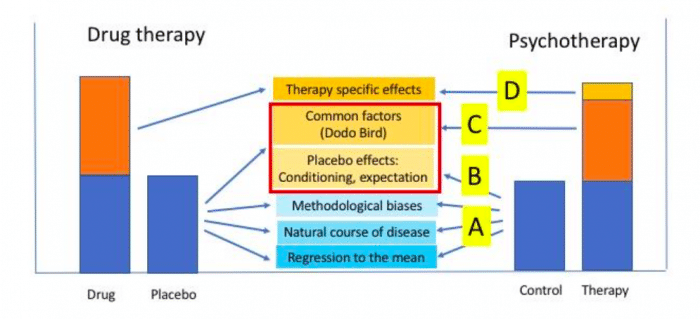
Credit: Paul Enck
In a drug trial, controlling the placebo effect is much easier than in psychotherapy — just use a placebo pill that looks like the real drug but does not contain the active pharmaceutical compound, give half of the patient the placebo and the other half the drug, blind both patients and doctor who received what, and treat all patients equally. To know how good your drug is, you only need subtract the mean of one group (drug) from the mean of the other group (placebo): the difference is the “true” drug effect.
To do the same with psychotherapy, you would need a “placebo psychotherapy” and you need to blind patients and therapists — both are impossible to achieve. So how do we solve this puzzle? Similar as in all those cases of drug trials, where the requirements (indistinguishability of true and placebo therapy = blinding) are also difficult, such as when the drug produces large side effects, but the placebo much less, when providing a placebo is ethically questionable, or when there is — technically speaking — no placebo therapy as with psychotherapy, but also with physical therapy and alike. In all these cases, we have to search for alternatives to a placebo control arm.
So, here are some recipes (6-8), provided that these are ethically acceptable in the condition to be tested — in all cases, this requires approval from an ethics board:
- Provide the drug in doses below efficacy that still produce side effects
- Use another drug with similar side effects, but ineffective for the treatment tested
- Compare the new drug to “treatment as usual” or “best available therapy”
- Compare the new treatment to an approved therapy, but blinded (“double-dummy”)
- Put patients on a “waiting list” to receive the therapy later: controls spontaneous variation
- Offer patients a choice of two alternatives; those without a preference are randomized
- Compare different therapies in different clusters (e.g. hospitals, practices)
Some of these strategies have effectively been used also in psychotherapy studies to control for placebo effects: waiting list (WL) and treatment as usual (TAU) are among the most commonly-used control strategies in psychotherapy research (6). They either control for the global effect of any treatment provided (TAU) or for spontaneous variation of symptoms independent of a treatment (WL). An alternative is to remove one or more components of a specific psychotherapy, e.g. CBT, to see whether the efficacy overall declines, or replace single components or the entire communication by computer-based psychotherapy (9) that has been shown to reliably induce clinical benefits. Particularly, the inclusion of electronic mobile media (called eHealth and mhealth) and apps likely allows many novel control strategies, but probably also generate new “common factors” and “specific effects.”
One such strategy is based on patients’ preferences, called preference design (PD) (8), and has occasionally been used to compare drug treatment to psychotherapy in the same clinical condition, e.g. in depression in a clinical trial. Patients are offered a choice between two or more treatments, and only those without a preference are then randomized to one or the other. The PD allows to assess whether having chosen a therapy as compared to being randomized to it results in different efficacies. Therefore, it may also affect the comparison between the alternatives, another component of the “common factors” driving the placebo effect in psychotherapy. How many choices to offer for optimal treatment outcome has been investigated (10) but not yet for psychotherapy — probably “just right” is the best, as Goldilocks would say.
In the fairy-tale Goldilocks and the Three Bears, Goldilocks tasted the porridge of the three bears in their home, and found one too hot, one too cold, but the third received her approval because it was “just right.” This Goldilocks principle has made its way into science, from sociology to astrophysics, and certainly also into placebo research, as we have shown here.
This is part 6 of a series covering “placebo” provided by Paul Enck and Sibylle Klosterhalfen from the Tübingen University Hospital. Continuous updates on placebo research can be found at www.jips.online.
References:
- Rosenzweig S. (1936). Some implicit common factors in diverse methods of psychotherapy. Am J Orthopsychiatry 1936;6:412-5.
- Baskin TW, Tierney SC, Minami T, Wampold BE. Establishing specificity in psychotherapy: a meta-analysis of structural equivalence of placebo controls. J Consult Clin Psychol. 2003;71:973-9.
- Huibers MJH, Cuipers P. Common (Nonspecific) Factors in Psychotherapy. In: The Encyclopedia of Clinical Psychology, eds. R.L. Cautin & S.O. Lilienfeld. John WIley & Sons, 2015:681-6.
- Schedlowski M, Enck P, Rief W, Bingel U. Neuro-bio-behavioral mechanisms of placebo and nocebo responses: Implications for clinical trials and clinical practice. Pharmacol Rev. 2015;67:697-730.
- Grelotti DJ, and Kaptchuk T. Placebo by proxy. BMJ 2011;343:d4345.
- Enck P, Zipfel S. Placeboeffects in psychotherapy – a framework. (under review)
- Gold SM, Enck P. Hasselmann H, Friede T, Hegerl U, Mohr DC, Otte C. Control conditions for randomised trials of behavioural interventions in psychiatry: a decision framework. Lancet Psychiatry 2017;4,725-32
- Weimer K, Enck P. Traditional and innovative experimental and clinical trial designs and their advantages and pitfalls. Handb Exp Pharmacol. 2014;225:237-72.
- Fischer A, Schröder J, Vettorazzi E, Wolf OT, Pöttgen J, Lau S, Heesen C, Moritz S, Gold SM. An online programme to reduce depression in patients with multiple sclerosis: a randomised controlled trial. Lancet Psychiatry. 2015;2:217-23.
- Hafner RJ, White MP, Handley SJ. The Goldilocks placebo effect: Placebo effects are stronger when people select a treatment from an optimal number of choices. Am J Psychol 2018;131:175-84









