Many people are concerned about cholesterol because of high cholesterol levels in their blood. However, cholesterol is crucial for our life and participates in many events in the body. Most importantly, cholesterol is an essential component of cellular membranes, where it serves various functions.
The membrane is a fundamental structure that surrounds the whole cell and intracellular organelles providing sealed reaction compartments and a dynamic signaling interface between these compartments. Due to the uniqueness of its structure, cholesterol rigidifies membranes and, therefore, helps to create this semi-permeable barrier. Additionally, it is involved in trafficking and signaling events, but its exact role and mode of action in all these processes are not completely understood. Therefore, many research groups came up with the idea to utilize cholesterol-binding molecules or functionalized cholesterol analogs to study the distribution and function of cholesterol in the cell. However, the problem with the probes introduced so far is either their weak fluorescence or their low cholesterol analogy. Thus, the development of a functional molecule that reveals a high cholesterol analogy and excellent fluorescent properties is highly desired.
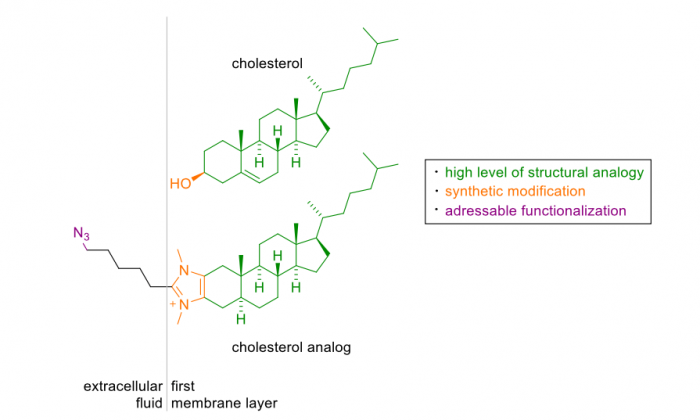
Researchers at the University of Münster in Germany approached this problem by synthesizing artificial analogs that were structurally based on natural cholesterol. The work was recently published in the journal Cell Chemical Biology. To obtain a cholesterol analog with membrane integration properties similar to the natural compound, they wanted to keep the structure as cholesterol-like as possible. Therefore, their synthesis efforts employed cholesterol as the starting material.
Three different compounds could be prepared by introducing a positively charged imidazolium salt replacing the small hydroxyl group of cholesterol. The imidazolium moiety is known to be stable in a cellular environment and could allow attractive interactions with the negatively charged phosphate moieties, which are part of the zwitterionic head groups of phospholipids present in cellular membranes.
Credit: Frank Glorius
Biophysical studies with model membranes were conducted to identify the compound that shows the highest cholesterol analogy in terms of membrane incorporation and fluidization. Among these compounds, one exhibited a very high cholesterol analogy and was therefore further functionalized. Many cellular studies rely on the use of fluorescence to visualize the localization and dynamics of components and processes they are involved in. It is an integral part of the design of the cholesterol analog that it can be derivatized/modified at a position that is not involved in lipid packing thus is located away from the membrane. Thus, a modification at this position should not significantly interfere with the function and properties of the analog.
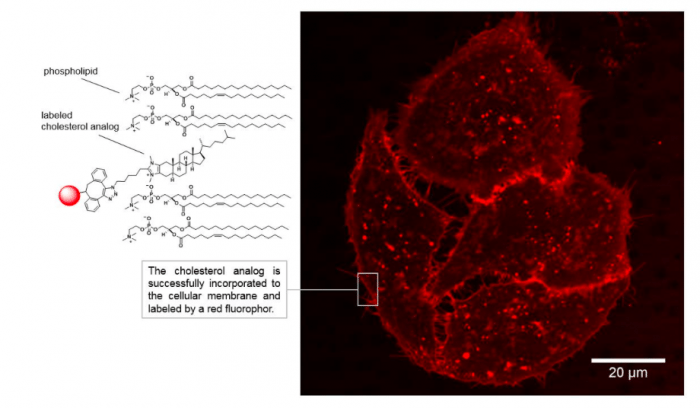
A functional azide group could be incorporated successfully at the imidazole ring of the compound allowing the on-demand coupling of a fluorescent molecule to the cholesterol analog via a click-chemistry approach. Click-chemistry is a powerful reaction used in biochemical experiments to label molecules. The set-up of the reaction is simple and requires functional groups on the target molecule and the labeling compound to “click” together. The fluorescently labeled cholesterol analog was next characterized in cellular experiments. The authors were able to observe the distribution and dynamic changes of the compound in live cell imaging experiments. Importantly, the membrane was not damaged by applying the synthetic analogs and control experiments again revealed the cholesterol-like properties when incorporated into cellular membranes.
Credit: Frank Glorius (left) and Anna LL Matos (right).
A great advantage of this molecule in comparison to known compounds is its addressable nature. Due to the integration of the functional group for click-chemistry, a large library of reporter molecules can be clicked on demand allowing the application in various experiments. Future studies will focus on the application of the molecules to understand the fundamentals of the role of cholesterol in membrane processes, which are crucial for biochemical and medical research.
These findings are described in the article entitled Addressable Cholesterol Analogs for Live Imaging of Cellular Membranes, recently published in the journal Cell Chemical Biology. This work was conducted by Lena Rakers, David Grill, Anna L. L. Matos, Stephanie Wulff, Da Wang, Jonas Börgel, Martin Körsgen, Heinrich F. Arlinghaus, Hans-Joachim Galla, Volker Gerke, and Frank Glorius from the University of Münster.
Reference:
- L. Rakers, D. Grill, A. L. L. Matos, S. Wulff, D. Wang, J. Börgel, M. Körsgen, H. F. Arlinghaus, H.-J. Galla, V. Gerke, F. Glorius, Addressable Cholesterol Analogs for Live Imaging of Cellular Membranes, Cell Chem. Biol. 2018, 25, 952 – 961.









