
Kombucha has received significant attention over last few years and has become an increasingly popular beverage with purported health-promoting properties. Kombucha is a traditional fermented drink that can be simply made by adding specific strains of bacteria, yeast, and sugar to tea extract. Allowing the mixture to ferment for a week or more results in a mildly acidic and mildly sweet carbonated drink.
While several studies have investigated the health benefits, toxicity, and bacterial compositions of kombucha, the actual concentration of key components in such beverages is still obscure. Ethanol is one of the crucial components believed to be present in low concentrations in kombucha. Quality control and regulatory demands necessitate strict and precise monitoring of the alcohol content in commercial products. According to the U.S. Alcohol and Tobacco Tax and Trade Bureau (TTB), any beverage containing more than 0.5% alcohol by volume (ABV) is considered alcoholic and should be consumed only by adults 21 years or older. In recent years, certain kombucha products have been found to exceed this limit, which translates to a need for an efficient method to monitor the ethanol levels in kombucha beverages.
Gas chromatography (GC) is a powerful technique for analysis of alcoholic beverages. GC is a separation technique which uses liquid coated on a solid support as stationary phase and gas as a mobile phase, and it is a universal technique for the analysis of volatile compounds. Coupling headspace sampling technique with GC facilitates extraction of volatile species from complex multicomponent samples such as kombucha. Separation of ethanol from other volatile components of kombucha was carried out on the recently developed ionic liquid-based WatercolTM 1910 column.
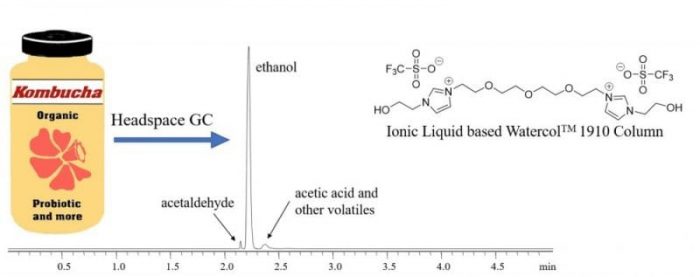
Figure 1. A chromatogram of a typical analysis of a commercial kombucha drink on WatercolTM 1910 column at 100 °C (left). Structure of ionic liquid-based WatercolTM 1910 column (right). Credit: the authors
Unlike conventional GC columns, the WatercolTM capillary columns are highly moisture-stable and their performance is not affected by the water present in kombucha samples. Analysis of kombucha products from five different brands revealed that these bottles contained 1.12-2% ABV, which was two-three times higher than the TTB regulatory limit for non-alcoholic beverages. Further, the ethanol content increased with time in these samples. Acetaldehyde and acetic acid were also identified in kombucha bottles.
Some kombucha manufacturers claim that store-bought kombucha products contain higher ethanol because the bottled product keeps fermenting while sitting on shelves. The effect of storage period on the ethanol concentration of kombucha products was examined under two different conditions; room temperature (22 °C) and refrigeration temperature (4 °C). A gradual increase in the ethanol concentration of kombucha drinks was observed for both batches. However, refrigeration helped in decreasing the rate at which ethanol accumulated in commercial bottled products. In both cases, the ethanol content seemed to maximize within ~14 days.
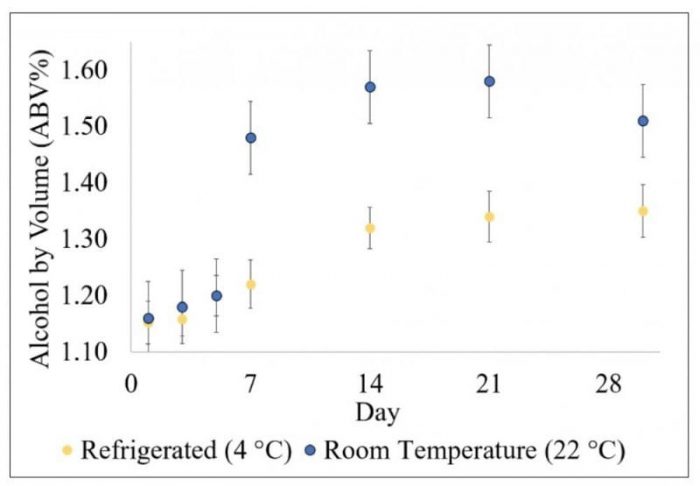
Figure 2. Effect of storage period on the ethanol content of kombucha. Credit: the authors
Several factors may impact the amount of alcohol in kombucha beverages, including but not limited to the microbial composition of kombucha culture, the initial sugar content, the time course of fermentation, and incubation temperature.
These findings are described in the article entitled Examination of the Varied and Changing Ethanol Content of Commercial Kombucha Products, published in the journal Food Analytical Methods. This work was led by Daniel Armstrong from the University of Texas at Arlington.









