
Published by Barbara Mulloy and Chris Rider
Imperial College London, Department of Medicine and the National Institute for Biological Standards and Control
These findings are described in the article entitled The localisation of the heparin binding sites of human and murine interleukin-12 within the carboxyterminal domain of the P40 subunit, recently published in the journal Cytokine (Cytokine 110 (2018) 159-168). This work was conducted by Pascale Garnier, Rosemary Mummery, and Christopher C. Rider from the Royal Holloway University of London, Mark J. Forster from the National Institute for Biological Standards and Control and Daresbury Laboratory, Barbara Mulloy from Imperial College London, Department of Medicine and Roslyn V. Gibbs from the University of Portsmouth.
Interleukin 12 and immune regulation
Evolution has gifted us a powerful immune system which is effective at protecting us from recurrent infectious diseases and also, as we have become more recently aware, cancers. The immune system has two arms, one being the production of antibody proteins by so-called B cells. The other is the cellular arm in which our T cells directly attack and destroy infected or abnormal cells. In most immune responses one or other arm is predominantly activated, so there is an early decision point as which route is to be followed.
The regulation of immune activity is highly complex, but one important mechanism is through a number of activating messenger proteins called interleukins. These are released by various white blood cells and function to activate particular cells within the immune system. We have been studying one such interleukin, interleukin 12 (IL-12), which is involved in initiating the cellular arm of immunity. Laboratory animals and human patients unable to produce IL-12 show persistent infections from those pathogens able to live inside cells due to a reduced ability to root out such infections by sacrificial destruction of infected cells.
IL-12 binds to heparan sulfate
Our immune cells are highly mobile, circulating through not just our blood vessels but also the lymphatic system. However, on activation, immune responses are highly focused on sites of infection and neighboring lymph nodes. Given that interleukins are mostly small, highly-soluble proteins which should be able to diffuse rapidly away on secretion, how is localized immune activation achieved? One explanation is that many interleukins bind to the highly acidic polysaccharide, heparan sulfate (HS). HS occurs as long unbranched chains, which are either tethered to the cell surface or found in the matrix which surrounds cells in the tissues. HS-binding proteins will, therefore, be constrained close to their sites of secretion and reside there at relatively high concentrations.
Previously, we found that IL-12 is among those interleukins which can bind strongly to HS. IL-12 is actually a relatively large interleukin containing two different subunits, which on the basis of size are called p35 and p40. We showed that it was the larger p40 subunit which was responsible for HS binding. This is significant because p40 is also a component of interleukin 23 (lL-23), which also is an immune-stimulatory protein, but one which underlies chronic autoimmune diseases. Blocking IL-23 activity is now used in the treatment of chronic autoimmune conditions such as psoriasis and Crohn’s disease. We predict that IL-23 binds similarly to HS though virtue of its shared p40 subunit.
The binding site for heparan sulfate (HS)
The binding of a protein to the highly-acidic HS depends on it having on its surface a suitable cluster of basically charged amino acid side chains. These can then engage in multiple interactions with the sulfate groups exposed along the HS backbone, although other protein-HS contacts may also participate in the binding. We noted, as have others, that within the sequence of p40 there is a short region which is highly enriched in basically charged amino acids. In the case of human p40, 6 basic amino acids occur within a stretch of 9 amino acid long stretch, a highly significant patch of such charged amino acids. We and others have predicted that this is likely to be at the center of the HS-binding site. The protein backbone of p40 is folded into three structural units, or domains, and this short basic sequence lies within the third of these.
More recently, we advanced our work on the interaction between IL-12 and HS. We generated a genetically engineered variant of IL-12 in which we removed the third domain of p40. In the absence of this domain, the variant IL-12 no longer bound to HS, establishing that this domain is indeed critical for this interaction. This provides for an even stronger focus on the basic sequence within this domain.
Since the chains of HS are bulky and have limited flexibility, to serve as HS-binding sites, basic amino acid clusters must be exposed and accessible on the surface of the protein. We found that the basic amino acid sequence within p40 is indeed suitably exposed forming a loop at one end of the D3 domain. We then used computer simulations of protein-HS binding which have previously proved reliable in predicting HS-binding sites in a variety of different proteins. Using these protocols to probe the surface of IL-12 in an unbiased manner did indeed predict binding to this particular loop sequence (see Fig. 1).
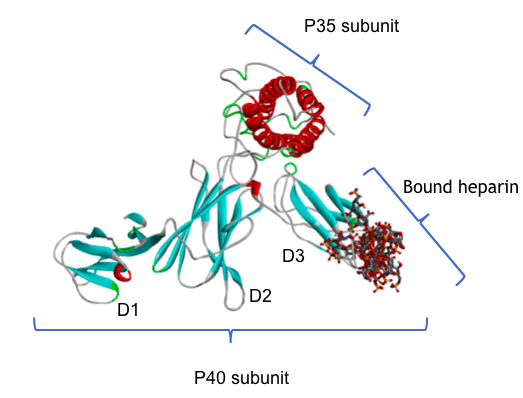
Figure 1: A molecular model of human IL-12 bound to a heparin fragment. Heparin, a closely related polysaccharide, is being used as a surrogate for heparan sulfate. The IL-12 protein is made up of the two subunits P40 and P35; the protein chain is shown as a ribbon, colored blue for strands and red for helical regions. P40 has three domains, D1, D2 and D3 as shown. Heparin fragments are shown in stick format, colored by element: grey for carbon, red for oxygen, blue for nitrogen and yellow for sulfur. The computational protocol used to make this model scanned the whole surface of the IL-12 protein and identified the loop at the end of domain D3 as the most likely heparin binding site. The diagram shows an overlay of multiple docking calculations, and the multiple results are closely superimposed.
The protein structure coordinates were taken from the Protein Data Bank (https://www.rcsb.org/) structure 3HMX.pdb, and the docking was performed using the ClusPro server with the heparin ligand option. Image courtesy Chris Rider
Both mouse and human forms of IL-12 bind strongly to HS, indicating that such behavior has been conserved across mammalian evolution. Examination of mouse IL-12 shows that it too has a basic residue cluster in a similar exposed position on the third domain. However, although overall mouse and human p40 sequences are very similar to each other, their basically charged loops are quite divergent. Our modeling studies, therefore, suggest mouse IL-12 must dock on to HS chains in a different orientation. Overall, we conclude in the case of IL-12 that binding to HS has been sufficiently important for its functioning that this property has been conserved, but that the details of exactly how this binding takes place can be flexible across the species.
When interleukins were first discovered as naturally occurring, powerful stimulators and manipulators of the immune system, there was great hope that we could exploit them in the treatment of diseases. Such anticipation has had to be tempered by complications, sometimes dangerous and even fatal, seen when they have been administered to patients. Amongst the complexities involved here, we can now appreciate that interleukins act naturally within the body in a localized manner, and that simply injecting them into the blood circulation is an inappropriate route of administration. We need to be better at mimicking, or at least taking into account, such localization of their activity within particular body tissues.









