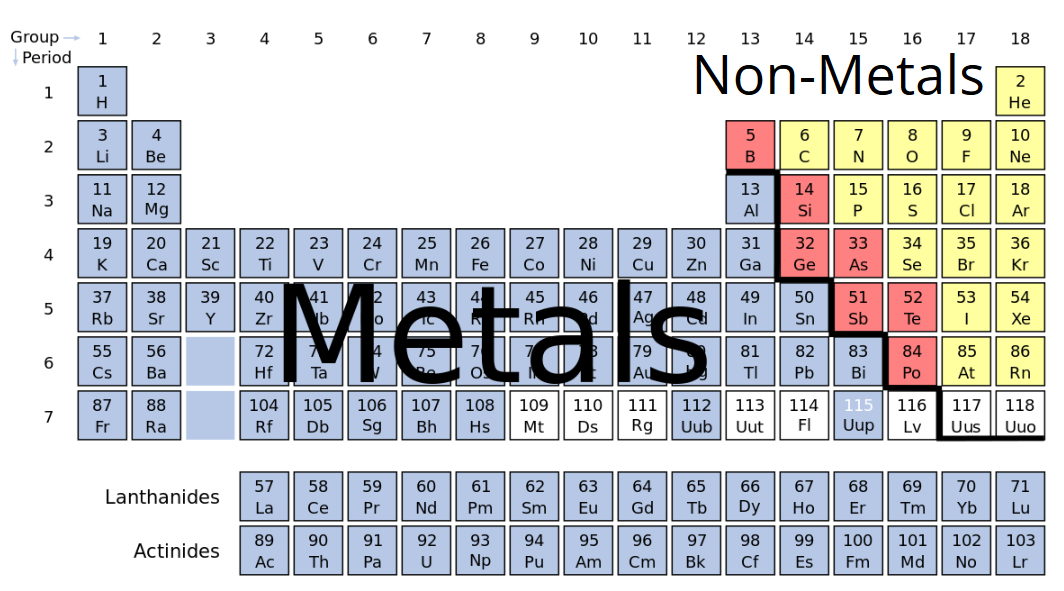
The metals list which makes up the periodic table includes iron, lead, gold, aluminum, platinum, uranium, zinc, lithium, sodium, tin, silver, etc.
The nonmetals list which makes up the periodic table includes hydrogen, helium, carbon, sulfur, nitrogen, oxygen, radon, neon, other halogens, and noble gases etc.
When we study the elements, it is important to know which elements are metals and which ones are not. If you are trying to learn to distinguish between metals and non-metals, a list and their uses is a good way to break them down and help memorize the difference between the two. The good news is that most elements are metals. A useful way to approach the study of elements is to distinguish whether they are metal or non-metal. Metals share some common properties. So, knowing what are those properties is a good way to begin our study.
What Is A Metal?
As stated in the introduction, most elements are metals or, at least, can be considered as such. Knowing which ones are or are not will help us to group them correctly. Before going over the complete list of metals, it is important that we define what a metal is. There are five different kinds of metals:
All metal elements are placed together in the periodic table.
We need less memorization – I never memorized the periodic table of the elements – I’ve never used it, and I’m a physicist! I can look it up. – Michio Kaku
There are several properties that make most elements metals. An element needs to share several of these properties in order to be considered a metal. It is important to become familiarized with these different properties. Not all properties are shared by all metals, but all elements that shared several of these properties can be considered metals.

“Periodic table blocks spdf (32 column)” by DePiep via Wikimedia Commons is licensed under CC-BY-SA 3.0
What Are The Common Properties Of Metals?
Most metals have a solid state when they are at room temperature. The only exception to this property, in fact, is mercury. Mercury is the only metal that is always liquid no matter the temperature. So it stays at a liquid stay even at room temperature. Generally, the melting point of metals is high.
- Another way to recognize a metal is that they tend to be shiny.
- Metals also tend to be a good conductor of heat and electricity. But have low ionization energies and low electronegativities. Another important property that many metal elements share is that they are malleable. This means that metals are relatively easy to be broken up into sheets.
- Also, most metals can be made into wire. This is what we know as being ductile.
- With the exception of potassium, lithium, and sodium, most metals have a high density.
- One of the common and, perhaps, most noticeable properties that most metal elements share is that they corrode when exposed to seawater or air.
- Finally, most metal elements lose electrons during reactions.
There is one non-metal element that can sometimes act as a metal. This is hydrogen that, when exposed to either extremely high or extremely low temperatures, can display some of these common properties.
The Complete List of Metals
Now that we have established the properties that make most elements on the periodic table into metals, we can now present the complete list of metal elements:
| Element | Symbol | Number In Periodic Table |
| Lithium | Li | 3 |
| Beryllium | Be | 4 |
| Sodium | Na | 11 |
| Magnesium | Mg | 12 |
| Aluminum | Al | 13 |
| Potassium | K | 19 |
| Calcium | Ca | 20 |
| Scandium | Sc | 21 |
| Titanium | Ti | 22 |
| Vanadium | V | 23 |
| Chromium | Cr | 24 |
| Manganese | Mn | 25 |
| Iron | Fe | 26 |
| Cobalt | Co | 27 |
| Nickel | Ni | 28 |
| Copper | Cu | 29 |
| Zinc | Zn | 30 |
| Gallium | Ha | 31 |
| Rubidium | Rb | 37 |
| Strontium | Sr | 38 |
| Yttrium | Y | 39 |
| Zirconium | Zr | 40 |
| Niobium | Nb | 41 |
| Molybdenum | Mo | 42 |
| Technetium | Tc | 43 |
| Ruthenium | Ru | 44 |
| Rhodium | Rh | 45 |
| Palladium | Pd | 46 |
| Silver | Ag | 47 |
| Cadmium | Cd | 48 |
| Indium | In | 49 |
| Tin | Sn | 50 |
| Cesium | Cs | 55 |
| Barium | Ba | 56 |
| Lanthanum | La | 57 |
| Cerium | Ce | 58 |
| Praseodymium | Pr | 59 |
| Neodymium | Nd | 60 |
| Promethium | Pm | 61 |
| Samarium | Sm | 62 |
| Europium | Eu | 63 |
| Gadolinium | Gd | 64 |
| Terbium | Tb | 65 |
| Dysprosium | Dy | 66 |
| Holmium | Ho | 67 |
| Erbium | Er | 68 |
| Thulium | Tm | 69 |
| Ytterbium | Yb | 70 |
| Lutetium | Lu | 71 |
| Hafnium | Hf | 72 |
| Tantalum | Ta | 73 |
| Tungsten | W | 74 |
| Rhenium | Re | 75 |
| Osmium | Os | 76 |
| Iridium | Ir | 77 |
| Platinum | Pt | 78 |
| Gold | Au | 79 |
| Mercury | Hg | 80 |
| Thallium | Tl | 81 |
| Lead | PB | 82 |
| Bismuth | Bi | 83 |
| Polonium | Po | 84 |
| Francium | Fr | 87 |
| Radium | Ra | 88 |
| Actinium | Ac | 89 |
| Thorium | Th | 90 |
| Protactinium | Pa | 91 |
| Uranium | U | 92 |
| Neptunium | Np | 93 |
| Plutonium | Pu | 94 |
| Americium | Am | 95 |
| Curium | Cm | 96 |
| Berkelium | Bk | 97 |
| Californium | Cf | 98 |
| Einsteinium | Es | 99 |
| Fermium | Fm | 100 |
| Mendelevium | Md | 101 |
| Nobelium | No | 102 |
| Lawrencium | Lr | 103 |
| Rutherfordium | Rf | 104 |
| Dubnium | Db | 105 |
| Seaborgium | Sg | 106 |
| Bohrium | Bh | 107 |
| Hassium | Hs | 108 |
| Meitnerium | Mt | 109 |
| Darmstadtium | Ds | 110 |
| Roentgenium | Rg | 111 |
| Copernicium | Cn | 112 |
| Ununtrium | Uut | 113 |
| Flevorium | Fl | 114 |
| Livermorium | Lv | 116 |
The Complete List of Non-Metals
Non Metals can be defined simply by having the opposite properties of metals. For example, they are not malleable, they have relatively low density, they are dull, and they are poor conductors of heat and electricity, etc.
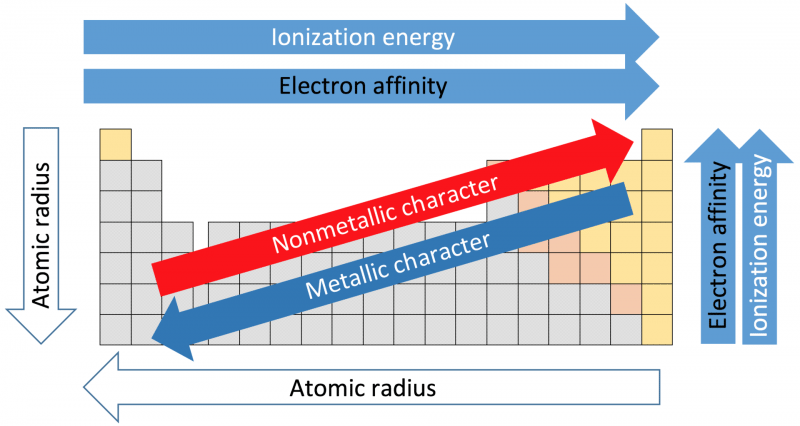
“Periodic trends” by Sandbh via Wikimedia Commons is licensed under CC-BY-SA 4.0 International
Here’s the full list if non-metals:
| Element | Element Symbol | Number In Periodic Table |
| Hydrogen | H | 1 |
| Helium | He | 2 |
| Carbon | C | 6 |
| Nitrogen | N | 7 |
| Oxygen | O | 8 |
| Fluorine | F | 9 |
| Neon | Ne | 10 |
| Phosphorus | P | 15 |
| Sulfur | S | 16 |
| Chlorine | Cl | 17 |
| Argon | Ar | 18 |
| Selenium | Se | 34 |
| Bromine | Br | 35 |
| Krypton | Kr | 36 |
| Iodine | I | 53 |
| Xenon | Xe | 54 |
| Astatine | At | 85 |
| Radon | Rn | 117 |
| Oganesson | Og | 118 |
We hope this list of metals and non-metals in the periodic table helps. Let us know in the comments below which one you were looking for in particular.









