
Welcome to those who do not believe in climate change and wish to start a friendly debate over the internet! Better yet, welcome to those that do not fully understand the phenomenon of climate change and how human activity contributes to the occurrence – and are curious to learn more.
And, best of all, welcome to those who are genuinely concerned about climate change and our impact causing the acceleration of it — and wish to share this article on their Twitter, Facebook, LinkedIn, etc. to educate their friends, families, and acquaintances as to the simplest of climate change components that is based in the basics of science.
Whichever camp you belong to, the intent of this article is to shed light on the fundamentals of how human-made activities impact the accelerated pace of global warming – otherwise known as climate change; while particularly focused on the physics (i.e. thermodynamics).
By now, you have likely heard the term “greenhouse gases” and understand that these are gases that enact a thermal trapping mechanism which increases the heat held within our environment. This heat/thermal energy, of course, comes courtesy of the fission/fusion reactor at the center of our solar system that we call the sun.
The most prevalent and well-known gases that fall within the umbrella category of greenhouse gases are that of carbon-dioxide (CO2) and methane (CH4). Both are linked very closely to human driven activities and technologies; carbon dioxide being linked to fossil fuels and methane to animal agriculture.
Certain properties related to these gases vary significantly compared to that of the most common gaseous compounds in our environment – i.e. oxygen (O2) which makes up approximately 21% of our atmospheric composition and nitrogen (N2) which makes up approximately 78% of Earth’s atmospheric composition.
The property that is most important to understand in relation to climate change and climate change science is simple – it is the property of specific heat. It is also the property that drives the greenhouse gas effect of retaining additional heat and converting that heat energy into greater temperatures.
So, what is specific heat?
I’m glad you asked.
Specific heat is defined as the amount of heat per unit mass required to raise the temperature of a substance, element, or compound by one degree – typically measured in Kelvin or Celsius. *It is important to note that the delta (or difference) in 1 degree Kelvin is equivalent to delta of 1 degree Celsius.
These specific heats change depending upon a number of variables – such as current temperature, atmospheric pressure, etc.
For the sake of simplicity, we will initially examine the various specific heats across all of these gases at 1 ATM (atmospheric pressure) and a temperature of 80 °F (26.67 degrees Celsius) which, is conveniently the same temperature in Austin, Texas today… in November.
All measurements of specific heat are in kj/(kg*K) or kilojoules per kilogram Kelvin. (kilojoules being a unit of energy, kilogram being a unit of mass, Kelvin being a unit of temperature)
- Oxygen: 0.918
- Nitrogen: 1.040
- Carbon dioxide: 0.846
- Methane: 2.26
At this temperature, carbon dioxide requires 7.84% less thermal energy than oxygen to increase in temperature by one degree Celsius (or 1.7 °F) per unit mass. In the same scenario, carbon dioxide requires 18.65% less thermal energy than nitrogen to increase temperature by one degree Celsius (or 1.7 °F).
Another way of looking at this; carbon dioxide is significantly easier to increase in temperature under the same amount of heat energy compared to that of oxygen or nitrogen.
An additional component to climate change is atmospheric thermal retention, or how much more negative energy is needed to decrease the temperature of a gas compared to another.
In this regard, methane is a gas that is much more difficult to cool down once heated compared to oxygen or nitrogen.
Using the specific heats referenced above, it is observed that methane requires 2.46X the amount of negative thermal energy to decrease one degree Celsius compared to that of oxygen, and 2.17X more negative thermal energy to decrease on degree Celsius compared to nitrogen.
Common & logical counter-argument
When presented with this information, skeptics intrinsically flip these perspectives around and say that carbon dioxide is easier to cool down by one degree Celsius and that methane is X% harder to increase temperature — so why worry? While technically true, it is paramount to remember that the Earth is always under constant heat/thermal energy courtesy of the fission/fusion reactor in our solar system. The inverse of the situations presented above does not occur frequently or at the same intensity across the globe. Thus the counter-argument, while seemingly logical at first glance, is null.
Another interesting characteristic with these specific heats is the concept of stability over temperature ranges or the fluctuation in the specific heat based on temperatures present. This also impacts climate change and atmospheric properties.
As mentioned at the start of the article, these specific heats change depending upon temperature – which then impacts the relation of the amount of heat energy required to increase the temperature of the gasses by one degree Celsius.
Let’s explore how differing present temperatures influence the specific heats of these gases.
For reference, we will measure the specific heat across a range of extreme temperatures that have occurred on Earth. On the lowest end, the chart reads from -99.4 °F and on the highest end, the chart reads 126 °F.
*Note, the lowest recorded temperature on Earth was -128.6 °F, measured in Antarctica in 1983. The highest recorded and confirmed temperature on Earth to date is at 129°F, which was measured in Kuwait in 2016.
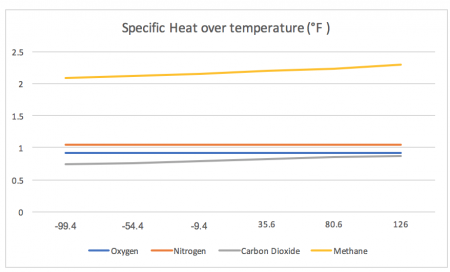
While the first chart may look as if it hardly changes, the second chart shows the true variation.
This second chart measures the percent change in specific heat between temperatures compared to the specific heat of each gas while at -99.4 °F.
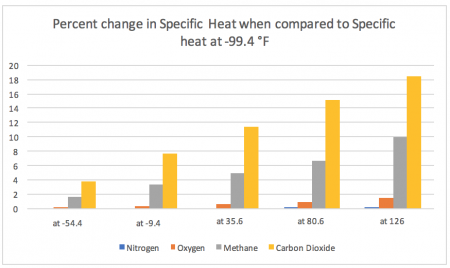
As you can see, the differences based on percentage change over temperature range is much easier to distinguish – and indicates the large variance in stability/consistency in specific heat for these gases across the temperature spectrum.
Observations
- The specific heat of nitrogen is incredibly stable across the temperature spectrum present on Earth, changing only ~0.096% when observed from -99.4 °F and 126 °F
- The specific heat of oxygen is very stable across the temperature spectrum present on Earth, changing only ~1.4% when observed from -99.4 °F and 126 °F
- The specific heat of both methane and carbon dioxide alter drastically across the temperature spectrum present on Earth, changing ~9.8% and ~18.5% respectively when observed from -99.4 °F and 126 °F
(These are the data points used to create these graphs)
| Nitrogen | Oxygen | Methane | Carbon Dioxide | |
| -99.4 °F | 1.039 | 0.91 | 2.087 | 0.735 |
| – 55.4 °F | 1.039 | 0.911 | 2.121 | 0.763 |
| – 9.4 °F | 1.039 | 0.913 | 2.156 | 0.791 |
| 35.6 °F | 1.039 | 0.915 | 2.191 | 0.819 |
| 80.6 °F | 1.04 | 0.918 | 2.226 | 0.846 |
| 126 °F | 1.04 | 0.923 | 2.293 | 0.871 |
*Source: compiled from various resources via Engineeringtoolbox.com
Key Takeaways/Reminders
- Earth is under a constant heat source (the sun).
- Carbon dioxide requires substantially less positive thermal energy to increase in temperature.
- Methane requires significantly more negative thermal energy to decrease in temperature once heated.
- Even at the hottest end of Earth’s current temperature range, carbon dioxide still requires 5.6% less thermal energy than oxygen to increase one degree Celsius and 16.25% less thermal energy than nitrogen to increase one degree Celsius.
- The hotter methane gas becomes, the more negative thermal energy it takes to decrease its temperature.
- The increased release of carbon dioxide and methane into the atmosphere at massive volume/scales from fossil fuels and animal agriculture disrupts the atmospheric specific heat stability that has enabled a climate range for humans to evolve to be the dominant cognitive species.
In short, climate change exists and manmade activity influences it. Understanding the specific heat differences in these gases is the foundation for understanding how mankind impacts climate change. We must also understand the scale at which these gases are released via human-driven activities – this will be the subject of future articles.
While the above simplifies the thermodynamics into digestible pieces, it is still clear that the continued increased release of these gasses has an impact on our atmosphere. Any denial of the basic, verified, and repeatable scientific observations and truths mentioned above is an indication of a refusal to accept a fact as fact.
Thanks for reading!
Was this helpful and does it make sense? Do you have a greater understanding of the subject than before? Are there any gaps in the explanation that would be beneficial to clarify? Are you still vehemently in opposition to scientific fundamentals and the ability to explain how people are causing climate change?
Please let me know what you think in the comments.
If you’ve found the insights shared here valuable and learned something new, please consider sharing with your friends and colleagues. The only way to progress is to have shared knowledge to adequately combat our negative influences in the world and to pave the way to a positive generational legacy.
——————————————————————————————————
Matthew A. Gallagher is an author, LSU Alumnus, and full-time digital business advisor.
His first book, ‘The Influence of Man’, introduces frameworks for analyzing man-made climate change factors based upon their gaseous output and associated thermal properties. He is a 2018 Forbes 30 Under 30 Nominee in the science category for his work in climate change science and basic mathematical analysis — both of which are presented in ‘The Influence of Man’.
References:
- Definition of Specific Heat: http://www.dictionary.com/browse/specific-heat
- Earth’s Atmospheric Composition: https://www.space.com/17683-earth-atmosphere.html
- Specific heat chart of oxygen https://www.engineeringtoolbox.com/oxygen-d_978.html
- Specific heat chart of nitrogen https://www.engineeringtoolbox.com/nitrogen-d_977.html
- Specific heat chart of carbon dioxide https://www.engineeringtoolbox.com/carbon-dioxide-d_974.html
- Specific heat chart of methane https://www.engineeringtoolbox.com/methane-d_980.html









