Those of us that have worn braces during childhood have probably never realized that this force on teeth evokes unique cellular responses of the cells that are located in the tiny gap of approximately 0.1 mm between the tooth and the bone socket into which the tooth is embedded. Teeth migrate through bone by induction of bone break-down at the “pull”-side and by inducing bone formation at the “push”-side.
The space between tooth and bone is the periodontal ligament, and, similar to muscle-to-bone ligaments that are more commonly known, fibroblasts from this ligament secure tight anchoring of teeth into bone.
Cells from the periodontal ligament can be isolated and propagated from extracted teeth, such as wisdom teeth and teeth that have to be extracted for orthodontic reasons. Researchers from The Academic Centre for Dentistry Amsterdam, The Netherlands, together with VU Medical Centre Amsterdam, have used this model to study both bone formation and bone degradation in a rare and complex bone disease — fibrodysplasia ossificans progressiva, FOP. The cells can be stimulated in either direction: towards bone formation, or towards providing the requirements for differentiation of osteoclasts, the bone degrading cells. The latter is achieved when co-cultured with peripheral blood (see figure).
As can be deduced from the name, FOP is a progressive disease that leads to ectopic bone formation with a fibrous component involved. The fibrous components are often ligaments and muscles which can transform into bone, thereby locking up joints and eventually leading to severely immobilized life and a shortened life expectancy. The mutation that causes FOP was described in 2006. A receptor of Bone Morphogenetic Proteins (BMPs), called ACVR-1, is mutated in all approximately 800 world-wide confirmed cases of FOP. In order to study FOP from patient-derived cells, however, clinicians are cautious to take biopsies from patients, since this may trigger bone formation. Therefore, patient-derived cell models are rare for FOP. And this is where cells from extracted teeth may become a valuable tool to study both bone formation and break-down since these are mandatorily extracted when the jaw joint of these patients lock.
The group in Amsterdam questioned whether both bone formation and bone break-down was altered in FOP patients using these cells. When using a TGF-beta inhibitor, that was previously shown to successfully inhibit increased bone formation in FOP, some of the bone formation parameters were slightly increased in FOP. However, the formation of osteoclasts, the cells that degrade bone, was inhibited especially in FOP- fibroblasts in the presence of the inhibitor.
The recent discovery of the ligand Activin A that specifically triggers bone formation by the mutated ACVR-1 in FOP patients by two different groups has excited the FOP research community and has evoked new hopes for a halt of heterotopic bone formation in FOP patients. Currently, a clinical trial has started based on preventing the binding of Activin A to the mutated ACVR-1. Also using the tooth-derived cell model, it was shown that Activin A especially triggers bone formation in FOP patients. Studies that investigate the role Activin A in osteoclast formation by these FOP patients-derived tooth-associated fibroblasts are ongoing.
The many of us that have undergone extraction of their wisdom teeth have probably never realized that instead of throwing this operational waste material in the bin, it can provide a wealth of information on fundamental processes of bone formation and bone break-down in health and disease. Ultimately, this could well be a model system to test medicine that on the one hand propagate bone formation and on the other hand inhibit the formation of osteoclasts.
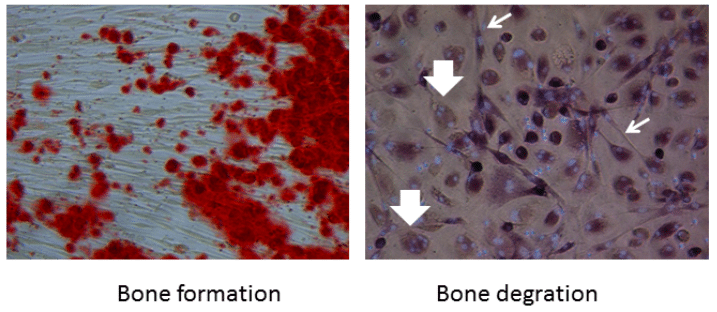
Figure 1: Fibroblasts from teeth contribute to bone formation (red dots in the left panel). Slender fibroblasts (light arrows) contribute to the formation of multinucleated osteoclasts (large arrows) that are formed from monocytes present in peripheral blood. Credit: Teun J. de Vries
These findings are described in the article entitled, Periodontal ligament fibroblasts as a cell model to study osteogenesis and osteoclastogenesis in fibrodysplasia ossificans progressiva, recently published in the journal Bone. This work was conducted by Teun J. de Vries, Ton Schoenmaker, Jolanda Hogervorst, and Siham Bouskla from ACTA, the University of Amsterdam and Vrije Universiteit Amsterdam, and Dimitra Micha, Tim Forouzanfar, Gerard Pals, Coen Netelenbos, E. Marelise W. Eekhoff, and Nathalie Bravenboer from VU University Medical Center, Amsterdam.









