
When it comes to the choice “left or right,” nature likes to stick to one. For instance, proteins are all “left-handed” — they’re made of “left-handed” fragments (peptides), which are composed of “left-handed” building blocks (amino acids). This is important for biological recognition and for the regulation of life processes, just like your left foot fits only your left shoe.
Indeed, cells often use proteins and peptides to speak to each other and do things such as movement, or transformation. For this reason, it is not uncommon that drugs are designed to mimic some of these peptides and/or to stop a protein from talking to others or from functioning.
Sometimes peptides can be used as drugs directly, but often there’s a problem: if they are “left-handed,” they’re quickly recognized by enzymes that cut them down to pieces, while if they’re all “right-handed,” then they don’t work because they don’t fit the “left shoes” in the cells. That is why, typically, drugs are synthetic molecules, but drug design starting from a peptide is not easy.
Cell messages can be written in words as short as three building blocks in a “tri-peptide,” which is much cheaper and simpler to make than a protein. Unfortunately, left-handed tripeptides don’t last long in a living organism because they’re immediately cut down to pieces by enzymes. However, it has been found that sometimes simple peptides can also have one “right-handed” building block, and this can even boost their activity.
Our research team at the University of Trieste, Italy, together with our collaborators have discovered how and why only tripeptides made of a combination of “left-handed” and “right-handed” building blocks at specific positions behave very differently than a simple left- or right-handed analog. These “ambidextrous” tripeptides can self-organize in water and form water channels and nanofibers that are also able to trap large amounts of water and yield gels that can be seen by the naked eye. The team has followed what happens when single molecules come together and give rise to the gels through a series of computational and experimental techniques and continuously through the scale sizes that span from single tripeptides to the gel we see by eye. In this manner, the properties of the final materials can be linked to the features of the tripeptides to assist in future new designs.
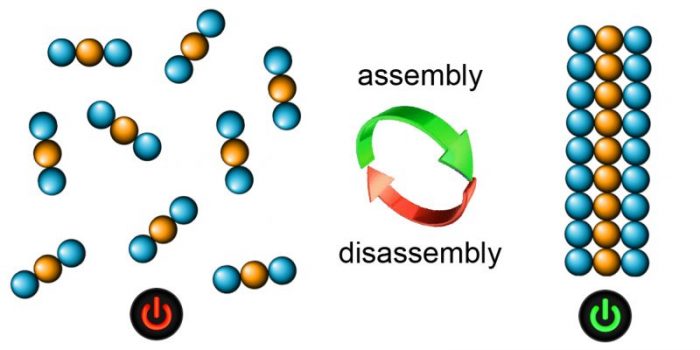
Image published with permission from Silvia Marchesan
These gels allow cell growth and proliferation over days, showing potential for biological applications. Importantly, these kinds of gels are held together by weak forces and therefore can easily be formed and disassembled on demand, for instance, with a change of pH or temperature.
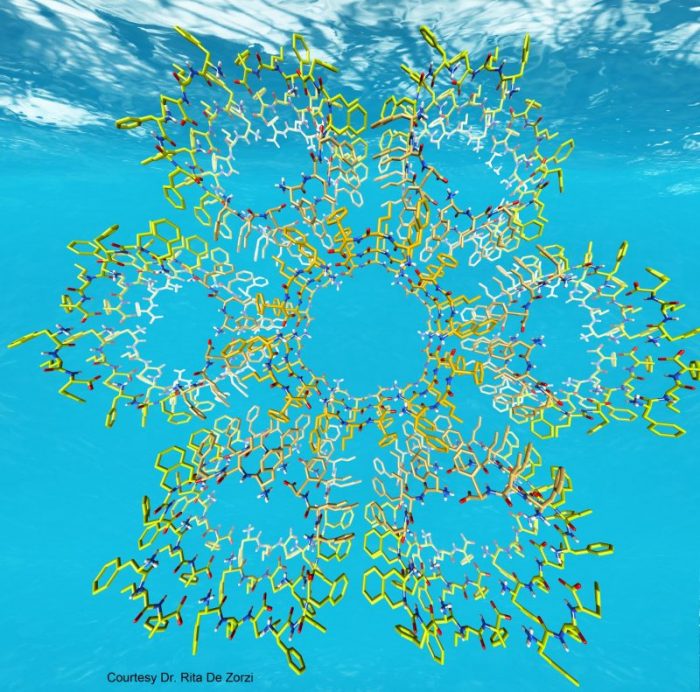
Image published with permission from Rita De Zorzi
Previous research by our team and collaborators had shown that systems of this kind can serve a function when assembled, so that the systems can be switched “on” and “off” as needed, and in the end, they can be disassembled to peptides and water, which do not harm the environment. A key feature is that these ambidextrous peptides are good at segregating water from dry regions, effectively creating different compartments. For instance, in one case, mild antimicrobial activity was reported for a gel, which could also be used for the sustained release of a poorly soluble drug, which participated in the self-organization process. In another case, a simple tripeptide could mimic a large enzyme and thus catalyze a reaction only when self-associated in nanofibers or in a gel, which could be disassembled and re-formed again.
Today, the performance of these systems still needs large improvements to have an impact on our lives, but the possible directions for this research are many and diverse. The team is currently studying whether these kind of systems can interfere with amyloid formation, which occurs in many diseases, such as Alzheimer’s. Other research lines focus on more fundamental questions, such as how to extend this approach to other peptides and how to use other triggers, such as “illumination,” to put them together or apart.
These findings are described in the article entitled Chirality Effects on Peptide Self-Assembly Unraveled from Molecules to Materials, recently published in the journal Chem. This work was conducted by Ana M. Garcia, Daniel Iglesias, Evelina Parisi, Caterina Deganutti, Rita De Zorzi, Mario Grassi, Michele Melchionna, and Silvia Marchesan from the University of Trieste, Katie E. Styan and Lynne J. Waddington from CSIRO Manufacturing, and Attilio V. Vargiu from the University of Cagliari.









