
With the increasing attention to environmental problems and the strong demand for clean and sustainable energy, it is becoming an important goal worldwide to manufacture an advanced energy storage device with high energy density, high power density, and long cycling stability. Recently, lithium-ion batteries (LIBs) have attracted much attention and achieved great success in smart laptops and light-electric vehicles.
Unfortunately, their large-scale application in long-range electric vehicles and hybrid vehicles is limited owing to the low capacity and bad rate performance of commercial graphite electrode material. Thus, it is highly urgent to research and develop advanced electrode materials with a higher capacity, longer cycling life, and better rate performance.
Recently, pursuing active, stable, low-cost, and well-designed electrode materials with superior rate capability and long-life cycling performance for LIBs plays an important role in improving the overall performance of LIBs. It is well known that transition metal phosphides (TMPs), especially FeP, have attracted much attention due to their high theoretical capacities, low cost, and non-toxicity. However, the batteries using FeP as anode materials showed unsatisfied cycling performance due to its intrinsically poor electrical conductivity and drastic volume change (>200 vol%) caused by the conversion reactions during lithiation and delithiation processes [1].
To date, several strategies have been used to improve the electrochemical storage performance for lithium of FeP. Firstly, designing nanoscale structure can shorten the diffusion distance of lithium ions and electrons and improve the active surface area of the electrochemical reactions. Secondly, combining FeP with carbonaceous materials can increase the electrical conductivity and buffer the volume changes. Thirdly, the electrode materials with hollow and porous structure can alleviate the volume variation, offer more active sites, and accelerate diffusion kinetic of ions and electrons. However, serious drawbacks such as the use of toxic precursors, as well as sophisticated and rigorous conditions make these strategies difficult to be applicable in practice [2]. Therefore, exploring novel and facile ways for the fabrication of carbon coated hollow and porous FeP structure still remains highly challenging.
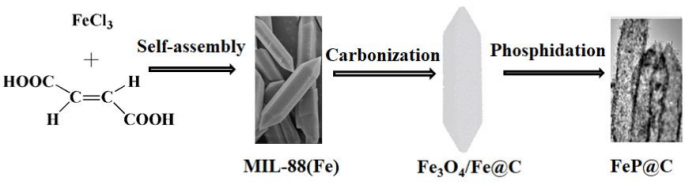
Figure 1 The illustration of the synthesis process of FeP@C from MIL-88(Fe). (Image courtesy of Xiaojie Zhang)
Herein, MIL-88(Fe), a typical MOF, has been used as an example to prepare ultrafine FeP nanoparticles encapsulated in a shuttle-like carbon polyhedral (Figure 1). The porous carbon layer not only benefits the electron conductivity as reported in the previous literature, but also prevents the growth and aggregation of ultrafine FeP nanoparticles during the preparation. From the structure characterization, the FeP@C displays a unique structure with ultrafine FeP nanoparticles distributed in the hollow and porous carbon matrix, which offers a large specific surface area and fast charge transfer ability, and alleviates volume change during cycling.
The FeP@C delivers a high maximum lithium storage capacity (902.4 mAh g-1 at 0.1 A g-1 for 100 cycles), superior rate capability (reversible capacity of 416 mAh g-1 at 5.0 A g-1) and long-life cycling performance (3000 cycles at 5.0 A g-1) owing to the good charge transfer ability and carbon coating as well as the large void space to alleviate the huge volume change of FeP during cycling. In addition, the excellent electrochemical performance is related to a significant contribution of pseudocapacitive behavior during the charge/discharge process, especially at high current density. The pseudocapacitive contribution percentages are 48.4%, 51.6%, 56.7%, 68.8%, 72.8%, 78.5%, and 81.5% at the scan rates of 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, and 1.4 mV s-1, respectively. The results show that the pseudocapacitive behavior for lithium-ion storage contributes significantly to the whole capacity, especially at high current density, and the high pseudocapacitive contribution should be attributed to small particle size with large specific surface area and high porosity [3].
The excellent electrochemical performance shows that the FeP@C can act as a high-performance candidate for the anode of LIBs and the current strategy should be also promisingly applied to synthesize other carbon-coated porous structures from MOFs for next-generation energy-storage application.
References:
- J. Cabana, L. Monconduit, D. Larcher, M.R. Palacín, Beyond intercalation-based Li-ion batteries: the state of the art and challenges of electrode materials reacting through conversion reactions. Adv. Mater., 2010, 22, E170-E192.
- P. Serp, P.alck, R. Feurer, Chemical vapor deposition methods for the controlled preparation of supported catalytic materials. Chem. Rev., 2002, 102(9), 3085-3128.
- T. Brezesinski, J. Wang, S.H. Tolbert, B. Dunn, Ordered mesoporous α-MoO3 with iso-oriented nanocrystalline walls for thin-film pseudocapacitors. Nat. Mater., 2010, 9, 146-151.









