
Biofouling has frequently been reported for any kind of material, from stone to metallic surfaces, on which many living organisms can grow to form biofilms because of exposition to the weather [Delgado, Borderie et al].
To overcome biofouling, the application of biocides in various forms (spray, compresses soaking, brushing, etc.) or the addition of antifouling agents to coating formulations were used to protect substrates against biological colonization.
In order to avoid excessive amounts of biocide which may pollute the environment and cause a macroscopic phase separation in the coating film in the case of incompatibility with the matrix, biocides can be encapsulated/entrapped in nanocapsules to control the release of bioactive species and to obtain a satisfactory long-action antifouling coating increase.
Among the natural product antifoulants (NPAs) the zosteric acid (ZA), or p-(sulfoxy) cinnamic acid (Fig. 1), first extracted from the Zostera marina, is proposed as a non- or less toxic alternatives to traditional biocides [1998 Directive of the European Parliament and of the council] whose antifouling capability as well as of its sodium salt was attributed to the sulfate group present in these compounds [Boopalan et al].
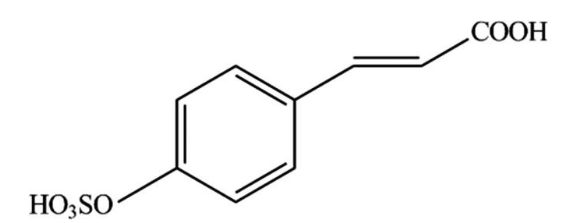
Fig. 1. Zosteric acid (p-(sulfo-oxy) cinnamic acid) structural formula. (Republished with permission from Elsevier)
Since the entrapment of NPAs in coatings formulations or their encapsulation to control the release have not been sufficiently addressed, the authors decided to create an innovative filler with controlled-release properties by encapsulating the not commercial zosteric sodium salt (ZS) in silica nanocontainers and to compare the results with nanocapsules containing sodium benzoate (BS), a commercial biocide used in many areas from cultural heritage to food preservatives, prepared in an analogous way.
The encapsulation procedure consisted of adding tetraethoxysilane to the oil-in-water miniemulsion formed by zosteric sodium salt dissolved in methanol, diethyl ether, and cetyltrimethylammonium bromide CTAB, NH3 in water; diethyl ether has the important role of the porogen, cosolvent, and template with CTAB for silica shell formation [Maia et al]. Sodium benzoate was analogously encapsulated using the same amount of biocide.
Incapsulation of the biocide in the nanoparticles loaded with by zosteric sodium salt (NC-ZS) and in the nanoparticles loaded with sodium benzoate (NC-BS) was revealed by FTIR analyses: the presence of ZS is confirmed by the bands observed at 1645 cm−1, associated with the stretching mode of C=O and the enlarged band centered at 1028 cm−1 due to the ν(SiOSi) which has a shoulder at 1220 cm−1, associated with νas(S O) signal, relative to the sulfate group present in the zosteric sodium salt. In the case of NC-BS, the encapsulation of BS is confirmed by the bands at 1601 cm−1 and 1406 cm−1, which are associated with the νas and νsym (COO-), respectively.
The amount of encapsulated biocide was evaluated via thermogravimetric analysis, 3.2% in the case of NC-ZS and 2.6% for NC-BS.
Morphological characterization was obtained from SEM and TEM analyses.
The empty silica nanocapsules (NC) (Fig. 2 a and b) consist of spherical particles with a regular shape with a porous structure with clearly distinguished pleats. According to previous studies, the spherical nanoparticles have a hollow nature, as revealed by the contrast between the dark edge and the pale center [Chen et al].
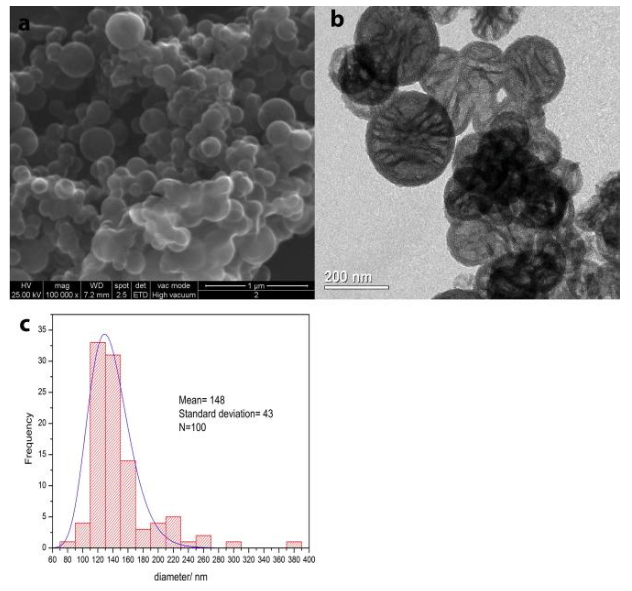
Fig. 2. (a) SEM and (b) TEM images of the empty silica nanocapsules (NC) and (c) histograms with size distribution obtained with ImageJ. (Republished with permission from Elsevier)
The nanocapsules loaded with ZS present analogous shape, but different size (Fig.3).
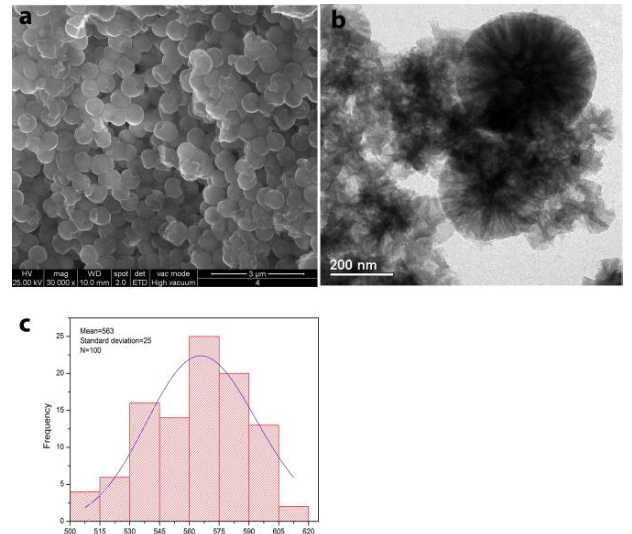
Fig. 3 (a) SEM and (b) TEM images of the silica nanocapsules loaded with the zosteric sodium salt (NC-ZS) and (c) histograms with size distribution obtained with ImageJ. (Republished with permission from Elsevier)
While NC have a size distribution centered at 148 nm with a standard deviation of 43 nm for 100 measurements, as reported in Fig. 2c., containers loaded with ZS seem to be larger than the empty ones (Fig. 3a and b), resulting in a size distribution centered at 563 with a standard deviation of 25 for 100 measurements (Fig. 3c).
In the case of NC- BS no significant variation in shape as well as in the size distribution of empty capsules was observed (Fig. 4a and b). The NC-BS have a size distribution centered at 164 nm with a standard deviation of 16 nm for 70 measurements (Fig. 4c). Due to the organic residual, the nanoparticles of both samples appear agglomerated (Figs. 2–4).
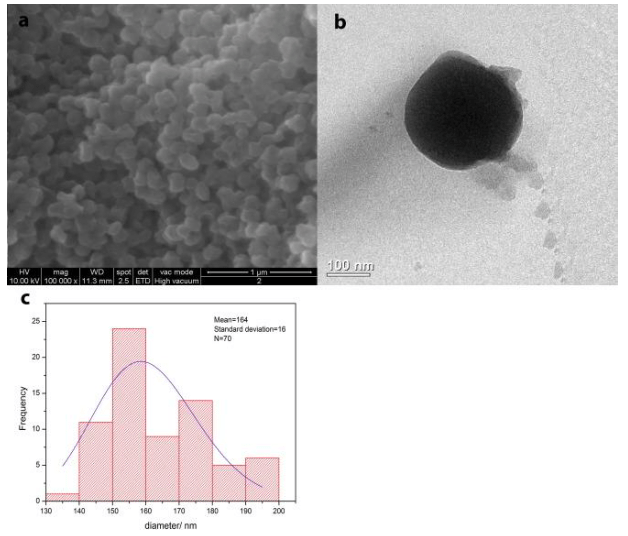
Fig. 4 (a) SEM and (b) TEM images of the nanocontainers with BS (NC-BS) and (c) histograms with size distribution obtained with ImageJ. (Republished with permission from Elsevier)
A successive research step was the study of controlled release of ZS and BS from the silica nanocontainers dispersed in ethanol by means of UV–Vis spectrophotometry at different times.
The releasing process of ZS initially proceeded fast then gradually slowed and leveled off after 22 ; 52% of ZS was released, and a fraction of the biocide is released until equilibrium between biocide in the nanocapsule and in the ethanol phase is reached. In the case of BS, the process does not level off after 50 h, and after 1 h the 34% of BS was released into ethanol. The differences in the release profiles of biocides are probably due to their different solubility in ethanol.
By studying the release of a sample, in which methyl zosteric ester (EZS) [Villa et al.] was physically adsorbed on silica which evidenced that the cumulative release of EZS was 97%, after 1 h and considering the thorough washing procedure of NC-Zs and NC-Bs with water, we can deduce that BS and ZS encapsulated by one-step miniemulsion process were mostly allocated in the core or in the shell structure of nanoparticles providing a good prolonged release.
We can, therefore, conclude that we were able to encapsulate for the first time the zosteric sodium salt in silica nanocapsules combining two innovative research lines: the use of environmentally-friendly anti-biofouling agent and the one-step encapsulation method. According to the literature data, the release results indicated that the encapsulation and controlled release of the organic molecule has been realized.
The perspective of integrating the nanocontainers synthesized in this work in a more complex antifouling coating system, namely super coating, will be interesting in order to evaluate the effectiveness of free zosteric sodium salt as well as encapsulated in silica nanocontainers in water-based and solvent-based coatings.
These findings are described in the article entitled Incorporation of the zosteric sodium salt in silica nanocapsules: synthesis and characterization of new fillers for antifouling coatings, recently published in the journal Applied Surface Science. This work was conducted by Ludovica Ruggiero and Elisabetta Zendri from the Università degli Studi Roma Tre and the Università Ca’ Foscari, and Laura Crociani, Naida El Habra, and Paolo Guerriero from ICMATE, CNR, Padova.









