
Infection by herpesviruses leads to many human diseases, ranging from a cold sore, chickenpox, to cancer. Human cytomegalovirus (HCMV) is a member of the β-herpesvirus subfamily of the Herpesviridae, with a high seroprevalence in the global human population via direct contact with bodily secretions, ranging from 50% in industrialized countries to as high as 99% in resource-poor communities and developing countries.
HCMV is the leading viral cause of birth defects and is often responsible for life-threatening complications among immunocompromised individuals. Like other human herpesviruses, HCMV is never completely cleared and remains latent throughout the life of the host once infected. Current anti-HCMV therapies are toxic to neonatal patients and can fail due to the development of antiviral resistance. As such, the rational design of vaccines and new therapeutics against HCMV is a public health priority.
HCMV exemplifies the canonical architecture of herpesviruses, with an icosahedral capsid enclosing a double-stranded DNA (dsDNA) genome, a partially-ordered tegument layer, and a pleomorphic envelope bearing glycoproteins essential for host-cell entry. Among the tens of glycoproteins, three — glycoprotein B (gB), which functions as a fusion protein, and glycoprotein H/glycoprotein L (gH/gL) complex, which functions as a receptor-binding protein — are conserved across all herpesviruses, forming the core viral fusion machinery. Moreover, the fusion glycoproteins are targets of neutralizing antibodies and represent exciting potential candidates for the rational design of new anti-HCMV therapies to prevent or disrupt viral cell entry.
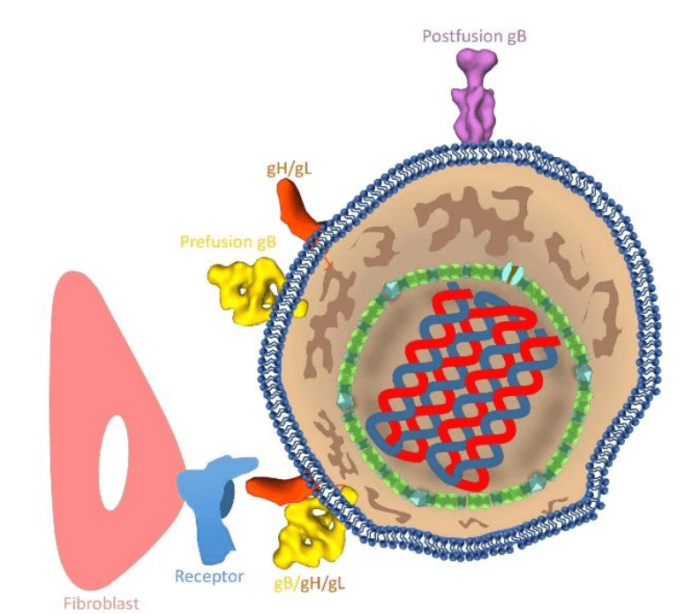
Schematic illustration of an HCMV (right) infecting a host cell (left). Yellow: prefusion gB; purple: postfusion gB; orange: gH/gL; pink: host cell; blue: receptor on host cell. Credit: Jiayan Zhang
Unfortunately, progress has been hindered by a shortage of structural information of these proteins, particularly in their native environment of an infectious virion. HCMV is the most genetically complex and, structurally, the largest among all human herpesviruses, with a genome of 235 kb and with a size exceeding 2000Å. The unusually large size of HCMV, its pleomorphic envelope, the irregularly organized proteins, and the metastable nature of the gB all pose significant technical challenges to high-resolution structural characterization. Consequently, the mechanism underlying the process of receptor-triggered membrane fusion remains poorly understood for not only HCMV but also for other herpesviruses.
To take a closer look at the fusion process, we employed the cryo-electron tomography (cryoET) method of combining Volta phase plate, direct electron detection, an energy filter, and subtomographic averaging. CryoET is a state-of-the-art imaging technique for high-resolution (1~4nm) three-dimensional (3D) view of samples. HCMV virions were collected from the cultured human fibroblasts, rapidly frozen in vitreous ice, and imaged in Titan Krios, the world’s most powerful transmission electron microscope. Just like the CT scan of the human body in clinical medicine to visualize our internal structures, here, HCMV virus samples are tilted and recorded every 2° or 3° tilting angles; a series of 2D images (2D projections) are collected and then aligned to produce a 3D reconstruction. The excellent contrast of the reconstructed images afforded by these cutting-edge technologies enabled us to identify and pick differently-shaped molecules. By averaging hundreds of these picked molecules, the signal-to-noise ratio of the structures of these molecules is improved sufficiently to resolve their domains.
In our study, we resolved two different conformational states of HCMV gB (303 kD) on native virions. About 21% of the gB trimers has the shape of a columnar tree and matches the known crystal structure of gB in the postfusion conformational state. The predominant (79%) gB trimers are in the prefusion conformational state and each has a shape of the Christmas tree. Moreover, we also observed complexes that are likely prefusion gB interacting with gH/gL. Integration of these structures and our knowledge of class III viral fusion proteins has led to a working model of HCMV fusion: gH/gL receptor-binding triggers conformational changes of gB endodomain, which, in turn, triggers two essential steps to actuate virus-cell membrane fusion — exposure of gB fusion loops and unfurling of gB ectodomain.
These findings are described in the article entitled Different functional states of fusion protein gB revealed on human cytomegalovirus by cryo electron tomography with Volta phase plate, recently published in the journal PLOS Pathogens.









