
As soon as lunar basalts became available for study, it was recognized from the absence of hydrated minerals from them, and the presence of metallic iron in them, that they were much drier and more reduced (oxygen-poor) than terrestrial basalts. Knowing just how reduced they are would improve our understanding of the composition of the Moon and the building blocks that went into forming it, and therefore even its origin. One way to investigate this is through the determination of the oxidation states of elements that can occur as either reduced or oxidized species in lunar rocks. Examples of such elements are titanium (can occur as Ti3+ or Ti4+), chromium (Cr2+ or Cr3+), and vanadium (V2+ or V3+). In contrast, in terrestrial rocks, Ti and V are tetravalent and Cr is trivalent.
We are conducting the first systematic study of the valence of these elements (actually, cations) in lunar rocks, using a technique called X-ray absorption near-edge structure spectroscopy, or XANES. Each element has a strong absorption edge related to the energy needed to excite its electrons. At slightly lower energies, in the “pre-edge” region, there are peaks that vary quantitatively in energy and intensity with the valence of the element. This relationship can be calibrated with measurements of standards, and then valences in unknowns can be determined. In addition, for titanium, the coordination environment can be determined as well, yielding proportions of Ti in four-coordinated (tetrahedral) vs. six-coordinated (octahedral) sites in the analyzed crystals.
This is important because typically, Ti is tetravalent (4+) and in pyroxene it can substitute for divalent (2+) cations in octahedral sites via a coupled substitution with aluminum, which enters tetrahedral sites to balance the excess charge:
Ti4+ + 2Al3+ ⇔ (Mg, Fe)2+ + 2Si4+.
Pyroxenes in which this is the dominant mechanism for the substitution of Ti and Al will, therefore, have atomic Ti/Al ratios of ~1/2. Trivalent (3+) Ti can enter pyroxene via a similar mechanism, requiring co-substitution with just one Al cation instead of the two required for charge-balance of Ti4+ substitution, so lunar pyroxenes with Ti/Al > 1/2 have long been inferred to contain trivalent Ti, which is not found on Earth. This inference is also partly based on the assumption that no Ti enters tetrahedral sites in lunar pyroxene.
Tetrahedral sites are usually mostly filled by Si4+ and Al3+. Actually, however, although Ti3+ cannot enter tetrahedral sites in pyroxene, Ti4+ can, especially if there is little Al available during crystal growth. So Ti3+ needs Al for coupled substitution, but Ti4+ may not. To investigate the effect of alumina activity on the coordination of Ti in lunar pyroxenes and to see whether lunar silicates contain Ti3+, for this study two aluminous basalts from Apollo 14, samples numbered 14053 and 14072, were analyzed. Sample 14053 is of special interest because it contains metal-rich regions not seen in other lunar samples, causing it to be called “the most reduced rock from the Moon”.
Valence Of Ti, Cr, And V In Pyroxene
Most analytical volumes excited by the X-ray beam during XANES analysis contain Ti, Cr and V cations in more than one oxidation state. The valence measurements obtained are the average valences in the analytical volumes. Representative spectra for each element in both pyroxene and olivine are shown in Fig. 1. The key features in these spectra are: for Ti, the intense peak at ~4970 eV, attributable to Ti4+ in tetrahedral coordination; for Cr, the low intensity of the peak at ~5997 eV, indicative of low Cr2+/Cr3+ ratios; and for V, a very low intensity of the peak at ~5468 eV, indicative of significant V2+ (relative to V3+) proportions. Most analyses have Ti valences within the analytical uncertainty of 4 and Cr valences between 2.75 and 2.85.
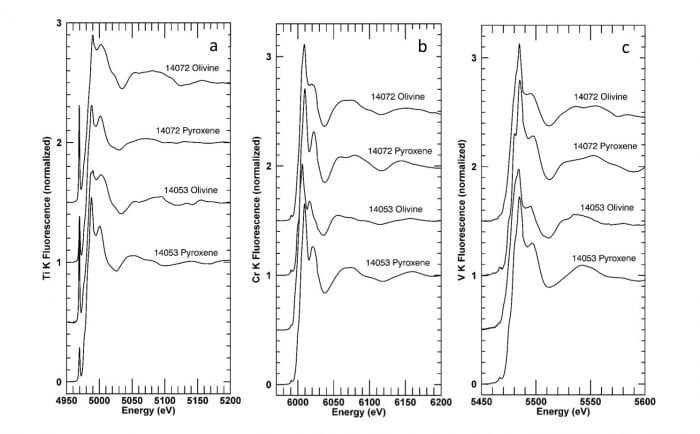
Figure 1. Representative absorption spectra for olivine and pyroxene in Apollo 14 samples 14053 and 14072. Reproduced from Meteoritics & Planetary Science.
In both samples, however, note that there is pyroxene that contains Ti3+ (i.e., the Ti valences are not within 1 standard deviation of 4) with Cr valences and FeO contents at the low ends of the observed ranges. There is also little correlation between Ti valence and Ti/Al ratio, as seen in Fig. 2. A surprising discovery is that most of the members of the low-valence, low-FeO cluster have relatively low Ti/Al ratios, ≤0.4, rather than ratios > ½, which are conventionally attributed to significant Ti3+ contents. Some of the pyroxene with Ti/Al > ½ also contains Ti3+, but all pyroxene with Ti valence within error of 4 has Ti/Al ≥ ½.
The available V valence data are somewhat limited. Except for one analysis that is within the error of 3, these results fall in a range from 2.63±0.05 to 2.85±0.05. These samples contain divalent and trivalent species of both Cr and V.
Valence Of Ti, Cr, And V In Olivine
In 14053, three large, magnesium-rich grains were analyzed. The Ti in them is all 4+ and mostly tetrahedrally coordinated, but the Cr in them, with valences of ~2.5-2.7, is reduced compared to the olivine in 14072. In the latter, up to ~20% of the Ti is trivalent. The V analyses show that the grains in 14053 have V2+ > V3+. In 14072, V valence increases with increasing fayalite (FeO) content. Substitution of Ti for Si in tetrahedral sites is the energetically preferred mechanism for incorporation of Ti in olivine formed under anhydrous (water-free) conditions, so the high proportions of tetrahedrally coordinated Ti found in lunar olivine are not surprising.
Ti And Al Substitution Into Pyroxene
Both samples contain some pyroxene in which ~20% of the Ti is trivalent and ~25% of the Cr is divalent. This pyroxene is among the most magnesium-rich in the samples, and it has low atomic Ti/Al ratios; both of these features indicate that it crystallized relatively early, before incoming of plagioclase, an Al-rich mineral. Uptake of Al by plagioclase led to later pyroxene having higher Ti/Al ratios, higher proportions of Ti in tetrahedral sites, and since the latter is all tetravalent, higher Ti4+/Ti3+ ratios.
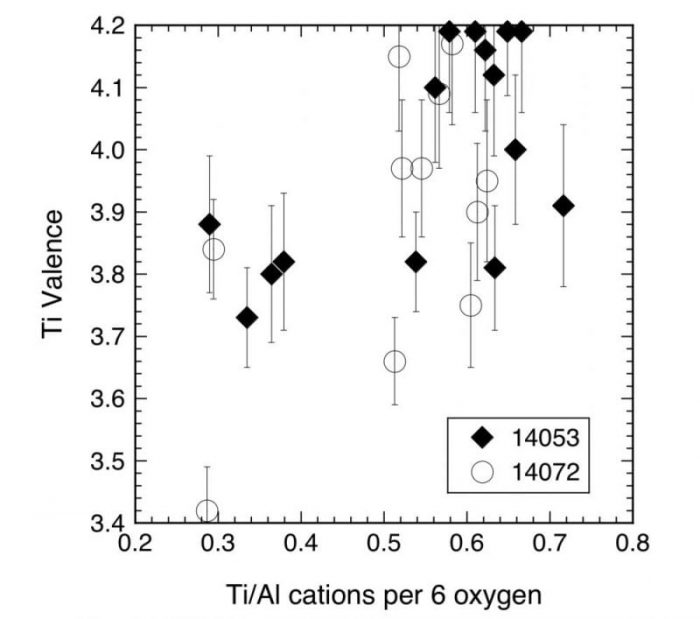
Figure 2. A plot of Ti valence against the Ti/Al cation ratio per formula unit for pyroxene. Grains with low Ti/Al contain Ti3+ while most of the other analyses are within the analytical uncertainty, indicated by the error bars, of a valence of 4 (i.e., having no Ti3+). Reproduced from Meteoritics & Planetary Science.
This is contrary to the long-standing inference that pyroxene with atomic Ti/Al >½ has trivalent Ti and that pyroxene with lower Ti/Al ratios does not. The observation indicates that the effect of co-crystallizing phases upon the melt bulk composition is greater than the Ti4+-2Al3+ substitution couple in the determination of site occupancies in pyroxene. Furthermore, in interpreting the Ti/Al ratios and the importance of the octahedral Ti- tetrahedral Al coupled substitution, one should consider only the octahedrally coordinated Ti. When the tetrahedral Ti component is subtracted from the total Ti, all analyses have Ti/Al cation ratios <½. Tetrahedral Ti has been reported in terrestrial diopside, some with Al2O3 and TiO2 contents similar to the present samples and Ti/Al >½. Naturally occurring, Al-free pyroxenes with two Si + Ti cations in the tetrahedral site per formula unit are also known.
These results illustrate the importance of alumina activity in the melt with respect to Al-Ti systematics. The preference for Al substitution over Ti in the tetrahedral site is demonstrated by a very Ti-, Al-rich pyroxene called fassaite, found in some meteorites, in which, despite much higher Ti contents (>10 wt% Ti oxides) than lunar pyroxene, no tetrahedral Ti is observed, along with much higher Al2O3 (>15 wt%) and tetrahedral Al contents. In fassaite the tetrahedral sites are completely filled by Si and Al, but that does not appear to be the case for lunar pyroxene.
The aluminous basalts such as 14053 and 14072 have higher bulk Al2O3 contents than typical mare basalts. This led to earlier incoming of plagioclase and higher modal amounts of plagioclase but did not yield higher Al2O3 contents in pyroxene.
The study, Valence of Ti, V, and Cr in Apollo 14 aluminous basalts 14053 and 14072 was recently published in the journal Meteoritics & Planetary Science.









