
In Stephen King’s 1981 novel Cujo (adapted to film in 1983), a boy and his mother are trapped in their car as a rabid Saint Bernard dog named Cujo, a good boy gone bad, aims to kill them. If you were a character in the book and survived this ordeal, what might be the possible consequences for you following such a brutal attack?
First, if you were bitten, you would need to get a vaccination as well as any additional medical care. Second, this event is likely to leave some emotional marks as well. Some people might start to feel less at ease in the company of dogs or feel intimidated by them. This fear might not only apply to Saint Bernards but also be generalized to other large dogs or dogs in general, large or small. The generalization will apply not only to stimuli (from Cujo to dogs in general) but also to contexts (from a car to parks, streets, and so on).
In extreme cases, intense experiences such as these might lead to the development of anxiety- and stress-related disorders, for instance, phobias or posttraumatic stress disorder (PTSD). The effects of stress hormones and additional modulators on the brain are the main reason why stressful events are so strongly remembered and easily generalized. In a recently published review, we show that stress itself can also aid in providing a solution.
Cortisol can improve exposure therapy
Let’s go back to the Cujo incident. Imagine that even after the scary experience, you still pay regular visits to your friend, who owns a dog. With multiple safe experiences with this dog, and perhaps other dogs in the park, you gradually learn that the equation “dog = danger” is not necessarily correct, and that “dog = safe” might apply as well. As a result, your fear response to dogs decreases. This learning is termed extinction learning and is the main underlying mechanism of exposure therapy, cognitive-behavioral psychotherapy aimed at the treatment of anxiety, PTSD, and additional disorders. But there’s a catch: since extinction learning does not erase the original fear memory, relapse is likely to occur. This, of course, is a major limitation for the long-term success of treatment.
In recent years, several research groups, ours included, have risen to the challenge, aiming to optimize extinction learning in various ways: cognitive/behavioral modifications (Schiller et al., 2010), brain stimulation (Dittert, Hüttner, Polak, & Herrmann, 2018), or pharmacological interventions, such as cannabinoids (de Bitencourt, Pamplona, & Takahashi, 2013). A particularly promising pharmacological candidate is cortisol, a glucocorticoids hormone. Studies have shown its beneficial effects in exposure therapy, e.g., for PTSD or phobias (de Quervain et al., 2011; Yehuda et al., 2015), as it leads to longer-term results and reduction in symptoms. But how does it work?
Cortisol creates stronger and more generalized memories
Cortisol is secreted from the adrenal gland in a circadian (daily) rhythm; its levels are highest shortly after we wake up, in what is termed the cortisol awakening response. In addition, cortisol levels sharply increase after we experience a stressful event. Here, cortisol has an important role in adjusting our physiological and behavioral response at the time of the event and returning the body to daily routine afterward.
One important role of cortisol is the modulation of learning and memory processes. In particular, cortisol leads to a memory consolidation mode, following which the memories of emotional events will become stronger and more persistent. At the same time, the retrieval of previous events is given a lower priority. This memory consolidation mode was suggested to explain the beneficial effects of cortisol in exposure therapy (de Quervain, Schwabe, & Roozendaal, 2017). However, previous studies did not take into account the question of the timing of cortisol administration (before, during, or after sessions?) and the effects of these variations on extinction vs. relapse.
This question has been the focus of our research group in the last several years. To answer it, we examined the effects of stress/cortisol treatment on extinction learning in healthy participants using two paradigms (contextual fear conditioning and predictive learning task) which allow the examination of renewal, that is, relapse after context change. Importantly, the timing of treatment varied between our studies, and so stress/cortisol was introduced either before extinction learning, after extinction learning, or before a retrieval test. Mainly, we aimed to reveal the right timing needed to achieve stronger and more generalized extinction memory.
Timing is (almost) everything
The results of our work are summarized in the STaR (Stress Timing affects Relapse) model (see Figure). In short, timing does make a difference. First, we found that stress/cortisol before extinction learning leads to a stronger, context-independent extinction memory, which is more resistant to renewal. This means that, under these conditions, extinction (i.e., safe) memories created in one context (e.g., in the clinic) will be generalized to other contexts (e.g., home, school, office).
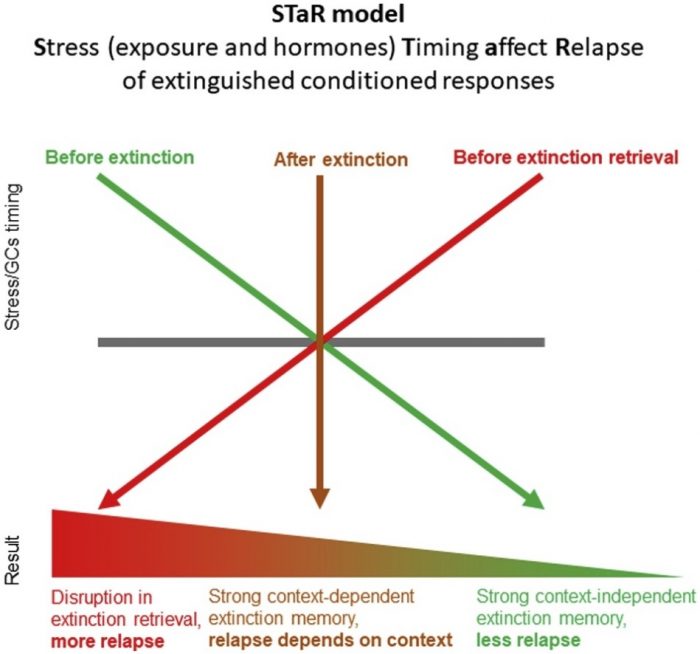
Image republished with permission from Elsevier from https://doi.org/10.1016/j.neubiorev.2018.12.029
The advantages of this for daily life improvements in anxiety and PTSD are obvious. Second, we found that stress/cortisol after extinction learning also enhances extinction, but in this case, memories are not easily generalized. Finally, exposure to stress/cortisol before a retrieval test led to an impairment in the retrieval of extinction memories, making relapse more likely to occur. Furthermore, we found that these timing-dependent effects of stress/cortisol on extinction memories are modulated by the amygdala, hippocampus complex, and pre-frontal cortex, brain areas that have a critical role in emotional learning and memory.
Our STaR model provides further support for the use of cortisol in exposure therapy and suggests that cortisol administration before extinction is the key for maximal benefits. Our findings may also suggest additional modifications of treatment, for instance, time-of-day variations that will harness the natural daily variations of cortisol (Lass-Hennemann & Michael, 2014). In addition, our work demonstrates the importance of further research on factors that might affect cortisol levels (either in general or in response to stress), such as circadian disruption (e.g., a result of shift work), chronic stress, sex hormones (e.g., during the female menstrual cycle), health status, and the use of additional medications. Taken together, our model shows that it is possible to fight fire with fire: Stress hormones, which initially led to the problem, can be used as part of the solution.
These findings are described in the article entitled How stress and glucocorticoids timing-dependently affect extinction and relapse, recently published in the journal Neuroscience & Biobehavioral Reviews.
Funding sources: Our work on memory extinction and reconsolidation is supported by Project A09 of the Collaborative Research Center 1280 “Extinction Learning” (PIs Christian J. Merz & Oliver T. Wolf).
Full citation:
- Meir Drexler, S., Merz, C. J., Jentsch, V. L., & Wolf, O. T. (2019). How stress and glucocorticoids timing-dependently affect extinction and relapse. Neuroscience & Biobehavioral Reviews, 98, 145–153;
https://linkinghub.elsevier.com/retrieve/pii/S0149763418306717
References:
- de Bitencourt, R. M., Pamplona, F. A., & Takahashi, R. N. (2013). A current overview of cannabinoids and glucocorticoids in facilitating extinction of aversive memories: Potential extinction enhancers. Neuropharmacology, 64, 389–395. https://linkinghub.elsevier.com/retrieve/pii/S002839081200247X
- de Quervain, D. J.-F., Bentz, D., Michael, T., Bolt, O. C., Wiederhold, B. K., Margraf, J., & Wilhelm, F. H. (2011). Glucocorticoids enhance extinction-based psychotherapy. Proceedings of the National Academy of Sciences, 108(16), 6621–6625. https://www.pnas.org/content/108/16/6621
- de Quervain, D. J.-F., Schwabe, L., & Roozendaal, B. (2017). Stress, glucocorticoids and memory: implications for treating fear-related disorders. Nature Reviews Neuroscience, 18(1), 7–19. https://www.nature.com/articles/nrn.2016.155?error=cookies_not_supported&code=7ef660c0-6a5a-4e9b-93ce-adee3b73edf9
- Dittert, N., Hüttner, S., Polak, T., & Herrmann, M. J. (2018). Augmentation of fear extinction by transcranial direct current stimulation ( tDCS ). Frontiers in Behavioral Neuroscience, 12, 1–16. https://www.frontiersin.org/articles/10.3389/fnbeh.2018.00076/full
- Lass-Hennemann, J., & Michael, T. (2014). Endogenous cortisol levels influence exposure therapy in spider phobia. Behaviour Research and Therapy, 60, 39–45. https://linkinghub.elsevier.com/retrieve/pii/S0005796714000965
- Schiller, D., Monfils, M.-H., Raio, C. M., Johnson, D. C., LeDoux, J. E., & Phelps, E. A. (2010). Preventing the return of fear in humans using reconsolidation update mechanisms. Nature, 463(7277), 49–53. https://www.nature.com/articles/nature08637?error=cookies_not_supported&code=04f9f334-3cf0-4ef6-832a-6d17f87c08ef
- Yehuda, R., Bierer, L. M., Pratchett, L. C., Lehrner, A., Koch, E. C., Van Manen, J. A., … Hildebrandt, T. (2015). Cortisol augmentation of a psychological treatment for warfighters with posttraumatic stress disorder: Randomized trial showing improved treatment retention and outcome. Psychoneuroendocrinology, 51, 589–597. https://linkinghub.elsevier.com/retrieve/pii/S0306453014003084









